Project Duration: 9 months
Partners: Gwalia Healthcare, Sky Medical Technology,
Aneurin Bevan University Health Board and Clinical Innovation Accelerator (Cardiff University)
Project aim: To undertake a clinical product evaluation of
the Geko™ device in patients with symptoms of COVID-19, and,
to enhance the device manufacturer’s capabilities, resilience
and sustainability within Wales

Project Overview
Venous thromboembolic diseases (VTE) are caused by a thrombus (blood clot) occurring in a vein. Patients are assessed for their VTE risk when they enter a hospital. High-risk patients and/or those who will be immobile for long periods of time are managed by prophylactic treatment.
COVID-19 is a highly thrombotic syndrome that leads to both micro and macrothrombosis and resultant multi-site embolism. Strict thromboprophylaxis has been widely indicated, but traditional anticoagulant therapy has, in some patients resulted in severe bleeding. This has led to an opportunity to explore
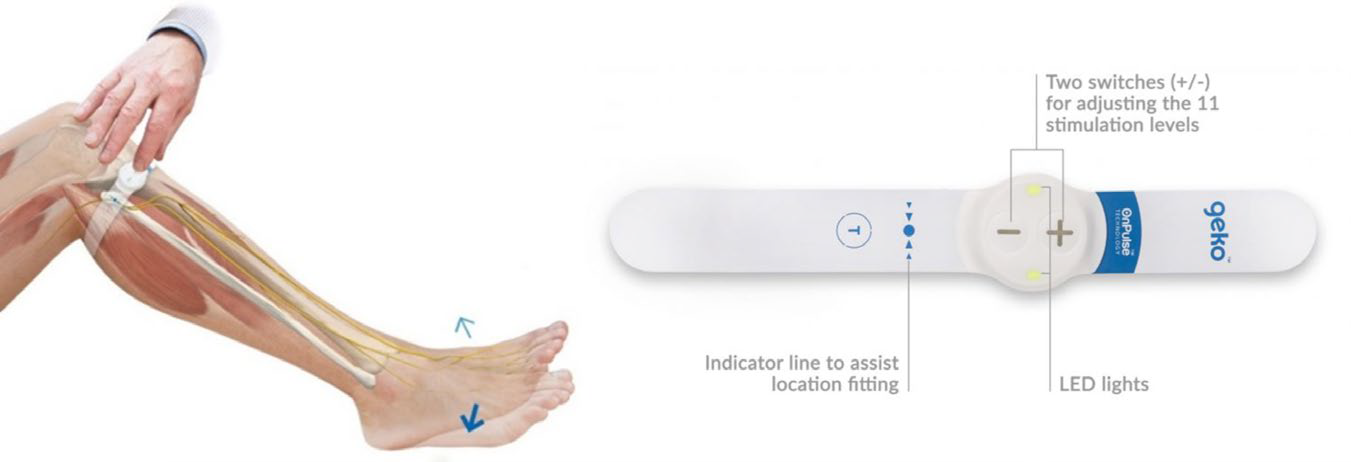
other options to support the management of VTE in this patient population. The geko™ is a wearable medical device that provides painless neuromuscular electrostimulation to the lower leg and increases blood circulation in immobile patients. It is manufactured in Wales and indicated for the prevention of venous thromboembolism (VTE), as well as the prevention and treatment of oedema.
The following project is being delivered by way of two parallel strands and focuses upon the geko™ device and its use with COVID-19 patients. Strand 1. A clinical product evaluation of the geko™ device in COVID-19 patients is being undertaken within Aneurin Bevan University Health Board (AB UHB).
This device is being added to standard care and may reduce the risk of clot formation in this patient group without the risk of severe bleeds.
Strand 2. Involves seeding the work around optimising geko™ device manufacturing capability in Wales. This work will explore new processes and strategies to enhance the Welsh manufacturer’s capabilities through a sustainable Welsh supply chain and opportunities to enhance the manufacturing line.
Accelerate's involvement
Accelerate is supporting the delivery of this project across these strategically aligned strands, with the intention that it will underpin a larger body of future work. This is being facilitated through the provision of Cardiff University academic expertise in manufacturing automation and supply chain resilience, dedicated project management and the support of nurse time to administer the device and collect data. This will be delivered in collaboration with the clinical and governance expertise of AB UHB, Gwalia Healthcare’s manufacturing experience and facilities for manufacturing the geko™ device, and
Sky Medical Technology with expertise in R&D, clinical product evaluations and manufacturing practices.
Expected outcomes
- Clinical data from the product evaluation
- Introduction of the geko™ device into ITU and acute respiratory unit for increased blood circulation, the prevention of venous thrombosis and, the prevention and treatment of oedema
- Consideration of changes to the clinical pathway for the management of COVID patients in the AB UHB
- Improved supplier assessment and procurement process with a focus on developing a resilient and sustainable Welsh supplier base
- Iterations to the automated manufacturing process for the geko™
- Case studies and peer-reviewed publications
- Seeding of future work
Future impact
- Changes to the clinical pathway for the management of COVID patients in the AB UHB
- Opportunities to explore the geko™ device use in specific patient cohorts across Wales
- Increased demand for the geko™ device
- Opportunities for further collaboration between project partners
- A definitively powered randomised controlled clinical trial.